
Heart of the development
In order to achieve a successful development, our electronic microscope plays a vital role.
It allows monitoring - real time – in order to observe the behaviour of different chemicals and reactions. This important information allows us to adjust and modify chemistry to achieve required results.
Solution from:
- Dendrite formation
- Gasification
- Cathode efficiency
- Zinc reversibility
- Recharging cycles

Dendrite formation
The development of dendrite is a serious problem, as it can lead to shorting between cathode and anode. Zinc air fuel cells and other batteries, like Lithium ion have this challenge. Dendrite development can lead to fires. Zinc air fuel cells do not create fire, but the energy is depleted by creating heat.
The pictures show the dendrite formation of the zinc, during energy production and changing to zinc oxide.
Picture with no dendrite inhibitor, shows sharp spikes that can penetrate the membrane and cause a short circuit.
Picture with dendrite inhibitor, shows reduction in sharp spikes, becoming rounder and reduced.


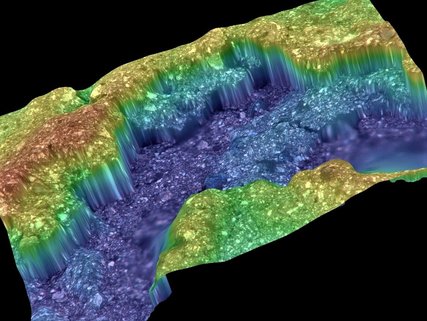
Cathode efficiency
The cathode is a vital part that allows energy production, energy density and reversibility of cycling the zinc oxide back to zinc.
Cathode material reacting on recharging
The recharging process changes the zinc to zinc oxide. Using energy, (that can be solar), the electrochemical process changes the zinc oxide back to zinc.
The pictures were taken after 40 recharges. The yellow/orange color shows the depth of zinc oxide penetration that will reduce the recharge times and energy output. It makes it more difficult for the electrolyte to penetrate. The blue color shows virgin cathode material. No relevant dendrite build up is visible. Cathode energy efficiency at this stage, is about 92% Further developments are in progress.

Zinc anode
The zinc anode produces the energy through reduction of zinc to zinc oxide. The zinc produces negative ions in the process. As the zinc oxidises, it increases in volume, creates dendrite and builds gas. All these actions are needing to be reduced and controlled, in order to allow a controlled, safe recharging process.
Gasification
The challenge is to reduce gasification, that leads to swelling of the fuel cell housing, that in turn could lead to leakage of electrolyte and drying out of the cell. In alkaline batteries this challenge is addressed by including space in the battery housing or including pressure release valves.